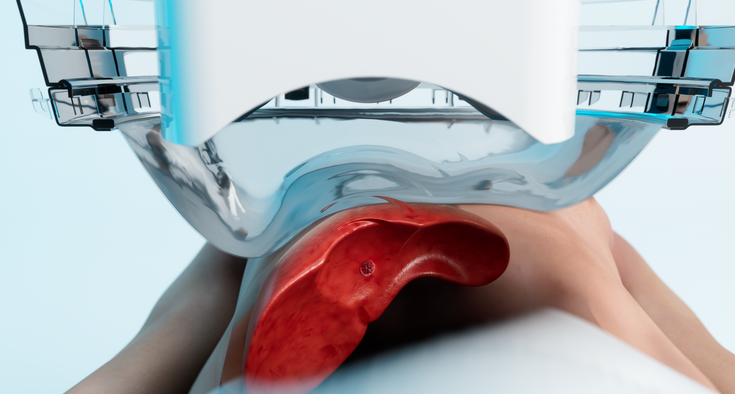
Novant Health Forsyth Medical Center in Winston-Salem is the first hospital in the Carolinas to offer Histotripsy, a non-invasive method to destroy liver tumors without surgery.
The ablation technique uses high-intensity ultrasound waves to shatter tumor cells while minimizing damage to surrounding tissues. Patients typically return home the same day or the following day.
“Where this technology really shines is its ability to fracture liver tumor cells without destroying the scaffolding of the tissues in which the tumor grows. The blood vessels and other structures remain intact and the patient does not require any sort of incision, which lessens hospitalization time.”
Sindram, a hepatopancreatobiliary surgeon who has treated cancers of the liver, pancreas and bile duct for more than 17 years, considers histotripsy a breakthrough treatment that will attract liver cancer patients throughout the Carolinas.
He has visited the Minneapolis-area headquarters of HistoSonics, makers of the histotrispy device, and attended conferences of early adopters of the technology in United States, he said. “I’m really excited for us to be on the forefront and help shape where this technology goes.”
Novant Health anticipates treating its first histotripsy patient in November.
How does the treatment work?
After ultrasound imaging to identify the exact location of the tumor, the physician uses a robot-controlled device to point an array of intersecting ultrasound waves at the lesion. Think of the movie “Ghostbusters,” Sindram said. “The individual rays from their guns were effective. But the moment they crossed beams, that’s when they became very powerful.”
The ultrasound waves create cavitation, or “bubble clouds” from gases in the targeted tissue.
The bubbles break down the tumor. “You can literally erase the cellular contents of an area,” Sindram said, “without removing the stiffer, more structural components of an organ.”
An MRI or CT scan is required a month after the procedure to confirm results. Most tumors can be destroyed in a single treatment, according to HistoSonics, though some cases will require multiple sessions.

What does the research suggest about who is a candidate for histotripsy?
Histotripsy has been studied in laboratories for several decades. Clinical trials began in the U.S. in 2018, and the procedure was approved by the U.S. Food and Drug Administration (FDA) in October 2023. Since then, more than 1,000 tumors have been treated in the United States with histotripsy. A 2025 study of histotripsy in the Annals of Surgery found that it produced a 90% local tumor control rate one year following treatment.
As long as the tumor is visible on ultrasound, the patient is a candidate. The physician can treat several liver tumors in one session.
How about side effects?
While any medical procedure has the possibility of side effects, Sindram said histotripsy has been remarkable in the lack of reported side effects seen with other ablative technologies, such as bleeding, leaks from bile ducts, infections or sepsis.
Do insurance companies cover the procedure?
Medicaid and Medicare have approved the treatment, and several major insurance companies have added this procedure to their coverage. Sindram anticipates that most insurers will cover the procedure as they learn about it. “Histotripsy will eventually become the standard of care,” he predicted.
Does histotrispy work on other types of tumors?
Histotrispy holds promise for kidney and pancreatic tumors. A kidney trial has concluded. The FDA’s assessment is due in fourth quarter 2025. Based on preliminary data, Sindram believes the FDA will approve the procedure for kidney tumors.
Pancreas tumors are among the most difficult to treat. Operations on pancreatic lesions are “some of the most damaging to patients and the most difficult to go through,” Sindram said.
“If we can use this technology to erase tumors out of the pancreas, that will be a shift in my profession. I’m thrilled to think about what might be possible.”
Safety and efficacy trials for pancreatic tumors are underway.
What has been the response from patients?
Patients where the technology has been available have embraced it as a less painful alternative to incisions and surgery. Major investors have also seen its potential, which has boosted its quick rollout.
The Novant Health Foundation has made this advanced care possible in the Carolinas by providing funding for the histotripsy device in Winston-Salem. Sindram hopes to offer the procedure at additional Novant Health facilities in the future.
Accepting Referrals for Histotripsy
Call 336-277-8804
Email HistotripsyReferrals@NovantHealth.org
Contributing Subject Matter Expert:
Our Connected Care Commitment
Our referral process is designed to save you time and improve patient care by reducing the obstacles you face every day – such as patients waiting weeks to be scheduled, repeated phone calls and unclear next steps.
- Save time. One centralized eFax handles referrals to all our North Carolina specialists.
- Accelerate care. Patients can get from referral to treatment with appointments available in as little as 48 hours.
- Higher patient satisfaction. Our optimized schedules and workflows create more appointment options for a smoother patient experience.
- Reduce administrative burden. Our process frees your team to focus on care, not coordination.
- 24/7 behavioral health. Patients can call 800-718-3550 anytime, with referral outreach within 48 hours.